iText 4.2.0 by 1T3XT [all data], Balk and Dong, 1964 (Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table). WebScience Chemistry Preparation of the pure silicon used in silicon chips involves the reaction between purified liquid silicon tetrachloride and magnesium. ga('set', 'dimension2', 'przemysl-budowlany|przemysl-farmaceutyczny|tworzywa-sztuczne|przemysl-energetyczny|fotowoltaika|branze-surowce-i-polprodukty-chemiczne|');
Fabric legend, testing details, and a caution from DuPont, National Oceanic and Atmospheric Administration. Purified tetrachlorosilane is used as a raw material for the production of optical fibres, silicon wafers and semiconductors. Chem., 1973, 11, 28-30. As we see, in the above structure, there are 4 single bonds used to connect all chlorine atoms(outer) to the silicon atom(central). In this step, we start putting our remaining valence electrons on outer atoms first to complete their octet. So, the AXN notation for the SiCl4 molecule becomes AX4N0 or AX4. 'produkt_segment':
National Institute of Standards and Reacts violently or explosively with water. Behavior in Fire: Contact with water in foam applied to adjacent fires will produce irritating fumes of hydrogen chloride. Permeation data for industrial chemicals is obtained per As the central atom always bonded with surrounding atoms, so, it has to share more electrons. China, the US and Japan account for about 80% of the total production of optical cable preforms, and in terms of consumption, China accounted for nearly 58% of the total optical fibre preforms in 2021. Also, by eliminating a number of devices, we limit the creation of waste, save energy and reduce the emission of greenhouse gases. It is a chemical formula for Silicon Tetrachloride. Answer: Silicon has vacant d orbital and thus it can expand its coordination number beyond four. J.
One of the most important ingredients in the production of optical fibres is high-purity silicon tetrachloride, usually 6N. It is miscible with toluene, benzene, chloroform, carbon tetrachloride, petroleum ether, ether and hydrochloric acid. So, put the silicon in the central position and spread all four atoms of chlorine around it.  Hybridization is defined as the mixing of two or more than two orbitals. This page provides supplementary chemical data on silicon tetrachloride. reduce burn injury during escape from a flash fire. How many function fdc9f57997a974af2a5836556275d729d(){ We will process your data until the communication with you is complete or until you object, unless the law obliges us to process it for a longer period or in case of potential claims, we will store it for the duration of the limitation period which is determined by law, in particular the Civil Code. Continue with Recommended Cookies. If you cant visualize the molecular geometry of SiCl4, then theoretically we can use an AXN method and VSEPR chart to determines its shape. are damaged, end user should worn as the outer foot covering. The product is a precursor in the process of pure silicon synthesis, used for the production of semiconductors and silicon anodes in lithium-ion batteries. Required fields are marked *. Database and to verify that the data contained therein have endstream Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. 2023 by the U.S. Secretary of Commerce the Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. It is used in smoke screens, to make various silicon containing chemicals, and in chemical analysis. The handling of this chemical may incur notable safety precautions. One of the most important significant role of lewis structure is that formal charge can be calculated using this structural representation. 9 Thus the Value of S is 4 in SiCl .It is also known All rights reserved. We process your data in order to send you a newsletter - the basis for processing is the implementation of our and third parties' legitimate interests - direct marketing of our products / products of the PCC Group . E. V. KartaevV. According to the VSEPR theory, when a central atom(silicon) is attached to four bonded atoms(chlorine) then an electron pair around the central atom repel each other as a result all corners atoms(chlorines) spread out as much as they can and takes the place where the repulsion is minimum and stability is much better. Be sure your answer has the correct number of significant digits. SiCl 4 can be synthesized by the chlorination of Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. ; Hadsell, E.M., SiCl4 Lewis structure comprises one silicon (Si) atom and four chlorine (Cl) atoms. Ingestion causes severe internal injury with pain in the throat and stomach, intense thirst, difficulty in swallowing, nausea, vomiting, and diarrhea; in severe cases, collapse and unconsciousness may result.
Hybridization is defined as the mixing of two or more than two orbitals. This page provides supplementary chemical data on silicon tetrachloride. reduce burn injury during escape from a flash fire. How many function fdc9f57997a974af2a5836556275d729d(){ We will process your data until the communication with you is complete or until you object, unless the law obliges us to process it for a longer period or in case of potential claims, we will store it for the duration of the limitation period which is determined by law, in particular the Civil Code. Continue with Recommended Cookies. If you cant visualize the molecular geometry of SiCl4, then theoretically we can use an AXN method and VSEPR chart to determines its shape. are damaged, end user should worn as the outer foot covering. The product is a precursor in the process of pure silicon synthesis, used for the production of semiconductors and silicon anodes in lithium-ion batteries. Required fields are marked *. Database and to verify that the data contained therein have endstream Silicon tetrachloride is a colourless liquid with a characteristic pungent odour. 2023 by the U.S. Secretary of Commerce the Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. It is used in smoke screens, to make various silicon containing chemicals, and in chemical analysis. The handling of this chemical may incur notable safety precautions. One of the most important significant role of lewis structure is that formal charge can be calculated using this structural representation. 9 Thus the Value of S is 4 in SiCl .It is also known All rights reserved. We process your data in order to send you a newsletter - the basis for processing is the implementation of our and third parties' legitimate interests - direct marketing of our products / products of the PCC Group . E. V. KartaevV. According to the VSEPR theory, when a central atom(silicon) is attached to four bonded atoms(chlorine) then an electron pair around the central atom repel each other as a result all corners atoms(chlorines) spread out as much as they can and takes the place where the repulsion is minimum and stability is much better. Be sure your answer has the correct number of significant digits. SiCl 4 can be synthesized by the chlorination of Hence, all chlorine atoms completed their octet comfortably as each one has 8 electrons for sharing. ; Hadsell, E.M., SiCl4 Lewis structure comprises one silicon (Si) atom and four chlorine (Cl) atoms. Ingestion causes severe internal injury with pain in the throat and stomach, intense thirst, difficulty in swallowing, nausea, vomiting, and diarrhea; in severe cases, collapse and unconsciousness may result.  Silicon tetrachloride is a colorless, fuming liquid with a pungent odor. shall not be liable for any damage that may result from (TRC) data available from this site, much more physical So, as per the VSEPR chart, if the central atom of a molecule contains 0 lone pairs and is cornered by four surrounding atoms, then the molecular shape of that molecule is tetrahedral in nature. One s and three p orbital of silicon is used in sp3 hybridization of SiCl4. personal protective equipment needed. }, Composition WebIn the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): [1] Si + 2 Cl2 SiCl4. [all data], Capkov and Fried, 1964
Silicon tetrachloride is a colorless, fuming liquid with a pungent odor. shall not be liable for any damage that may result from (TRC) data available from this site, much more physical So, as per the VSEPR chart, if the central atom of a molecule contains 0 lone pairs and is cornered by four surrounding atoms, then the molecular shape of that molecule is tetrahedral in nature. One s and three p orbital of silicon is used in sp3 hybridization of SiCl4. personal protective equipment needed. }, Composition WebIn the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): [1] Si + 2 Cl2 SiCl4. [all data], Capkov and Fried, 1964  Did you mean to find the molecular weight of one of these similar formulas?
Did you mean to find the molecular weight of one of these similar formulas?  SRD 103b Thermo Data Engine (TDE) for pure compounds,
Check the stability with the help of a formal charge concept. [all data], Parker and Robinson, 1927 Published By Vishal Goyal | Last updated: December 30, 2022, Home > Chemistry > SiCl4 lewis structure and its molecular geometry. application/pdf What are the differences between them? I am sure you will definitely learn how to draw lewis structure of SiCl4). The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR). National Ocean Service, WebSilicon tetrachloride (SiCl 4) is an inorganic compound. Now count the valence electrons we have used for making the above structure. You can learn more about how we process your data from our Privacy Policy. So you can see above that the formal charges on silicon as well as chlorine are zero. Total 24 lone pairs electrons and 8 bonded pairs electrons present in SiCl4 lewis dot structure. The bond angle of SiCl4 is 109.5 as the shape of its tetrahedral in nature and as per the VSEPR theory, a regular tetrahedral molecule holds that bond angle of 109.5. on silicon atom = (4 0 8/2) = 0, Shared pair electrons around chlorine = 2, F.C. Place remaining valence electrons starting from outer atom first. }, Function So it fulfills the octet rule and the silicon atom is stable. This section provides a listing of alternate names for this chemical, . Hence, only bonded atoms are used to determine the geometry of SiCl4. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. [all data], Sauer and Hadsell, 1948 [all data], Jain and Yadav, 1973 Technology, Office of Data We and our partners share information on your use of this website to help improve your experience. They have seven valance electrons and after bond formation with silicon atom, they have gained one electron from silicon as one valance electron from silicon is shared with each of the chlorine atom. Fibre optic technologies are becoming more and more popular every year. }, Segment 9. ; Yadav, O.P., If laboratory performance of fabrics, not complete garments, under High purity of silicon tetrachloride used in the manufacture of optical fibers. This problem has been solved! Data compilation copyright for the intended use. We will calculate the formal charge on the 4th step structure to verify its stability. But the four Si-Cl bonds are comparatively polar due to the electronegativity difference between silicon and chlorine atom (electronegativity of silicon and chlorine is 1.9 and 3.16 in Pauling scale). 'produkt_funkcja':
Kearby, K., on behalf of the United States of America. ["chloroalkalia","chlorosilany","produkty-specjalistyczne","dodatki-specjalistyczne"]
having technical skill for evaluation under their specific end-use It is broken down by water into hydrochloric acid with the release of heat. Other chemicals, such as germanium tetrachloride (GeCl4) and phosphorus oxychloride (POCl3), can be used to produce core fibres and outer coatings, or cladding, with function-specific optical properties. So now, you have to complete the octet on these chlorine atoms (because chlorine requires 8 electrons to have a complete outer shell). The lone pairs of oxygen in water are reacted with silicon and easily SiCl4 is hydrolyzed but CCl4 cant. conditions, at their own discretion and risk. Lower Explosive Limit (LEL): data unavailable, Upper Explosive Limit (UEL): data unavailable, Autoignition Temperature: data unavailable, Vapor Density (Relative to Air): data unavailable, Ionization Energy/Potential: data unavailable. All Photos (2) Silicon tetrachloride. (USCG, 1999). So there are no remaining electron pairs. WebSILICON TETRACHLORIDE At temperatures ranged within 1273-1573 K under atmospheric pressure oxygen actively reacts with SiCl4 with formation of silica and chlorine vapors, it Ind. by the U.S. Secretary of Commerce on behalf of the U.S.A. Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4 [ no lone pair on central atom of SiCl4]. SiCl4 is the chemical formula of silicon tetrachloride. on behalf of the United States of America. The molecular geometry of SiCl4 is tetrahedral as all four outer atoms(chlorine) are pushed away in all directions from the central atoms(silicon) because of repelling force occurs in electron pairs around the central atom. "), xl = x.length, s = ""; WebThe meaning of SILICON TETRACHLORIDE is a colorless fuming corrosive liquid SiCl4 made usually by heating silicon or silicon carbide with chlorine and used chiefly for These four bonds are directed towards the corner of a regular tetrahedron. In this article, we will discuss Silicon tetrachloride (SiCl4) lewis structure, molecular geometry, hybridization, polar or nonpolar, etc. Tetrachlorosilane; (Silicon chloride) (10026-04-7). The largest amounts of the produced tetrachlorosilane are used for the production of high-quality fumed silica. It is important to provide high-quality raw materials and semi-finished products. The derivatives are the starting point for the production of aerogels products with high market potential, strongly developing in the construction, automotive and pharmaceutical industries. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). SiCl4 is a nonpolar molecule in nature as its shape is highly symmetrical, also its central atom doesnt contain any lone pair, hence distortion of shape doesnt happen. Czech. An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles. However, the manufacturer does not guarantee the information and contents of this document are complete and accurate, and shall not be liable for the results of using them. WebMolecular weight calculation: 28.0855 + 35.453*4 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. Formula: Cl 4 Si. Total number of the valence electrons in silicon = 4, Total number of the valence electrons in chlorine = 7, Total number of valence electron available for the lewis structure of SiCl4 = 4 + 7(4) = 32 valence electrons [one silicon and four chlorine], 2. WebS o liquid: 239.7 J/(mol K) Heat capacity, c p: 145. Chim. WebSilicon tetrachloride. The next step is to calculate the number of bond (covalent or ionic) connectivity between the atoms. aluminized outer suit) garments should not knowingly enter an explosive This will be determined by the number of atoms and lone pairs attached to the central atom.If you are trying to find the electron geometry for SiCl4 we would expect it to be Tetrahedral.Helpful Resources: How to Draw Lewis Structures: https://youtu.be/1ZlnzyHahvo Molecular Geometry and VSEPR Explained: https://youtu.be/Moj85zwdULg Using the AXE Method for Molecular Geo: https://youtu.be/sDvecTjUZE4 Molecular Geo App: https://phet.colorado.edu/sims/html/molecule-shapes/latest/molecule-shapes_en.htmlGet more chemistry help at http://www.breslyn.orgDrawing/writing done in InkScape. responsibility to determine the level of toxicity and the proper Maxsperse 8900/100M is ChemstatG-118/42K is used as an internal lubricants in polyolefins (LDPE, LLDPE, HDPE, and PP). Building & ConstructionPharmaceuticalsPlasticsPower industry / PhotovoltaicsRaw materials and intermediates
All rights reserved. protective outer footwear and are not suitable as outer footwear. It is used as a raw material/intermediate in the production of metallurgical grade silicon, silica and other silicon-based substances. These pairs of electrons present between the Silicon (Si) and Chlorine (Cl) atoms form a chemical bond, which bonds the silicon and chlorine atoms with each other in a SiCl4 molecule. });
Will not burn under typical fire conditions. warfare agents is defined as the time when the cumulative mass which The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). Hello Everyone! Tetrachlorosilane is also of great importance in the electrochemical, photovoltaic and optical fibre industries. Data compiled as indicated in comments: WebSilicon tetrachloride 99.998% trace metals basis; CAS Number: 10026-04-7; EC Number: 233-054-0; Synonyms: STC,Tetrachlorosilane; Linear Formula: SiCl4; find Sigma-Aldrich-289388 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich We process your data in order to communicate with you and respond to your message.
SRD 103b Thermo Data Engine (TDE) for pure compounds,
Check the stability with the help of a formal charge concept. [all data], Parker and Robinson, 1927 Published By Vishal Goyal | Last updated: December 30, 2022, Home > Chemistry > SiCl4 lewis structure and its molecular geometry. application/pdf What are the differences between them? I am sure you will definitely learn how to draw lewis structure of SiCl4). The product is also used as a cross-linking agent in the processing of styrene-butadiene rubber (SBR). National Ocean Service, WebSilicon tetrachloride (SiCl 4) is an inorganic compound. Now count the valence electrons we have used for making the above structure. You can learn more about how we process your data from our Privacy Policy. So you can see above that the formal charges on silicon as well as chlorine are zero. Total 24 lone pairs electrons and 8 bonded pairs electrons present in SiCl4 lewis dot structure. The bond angle of SiCl4 is 109.5 as the shape of its tetrahedral in nature and as per the VSEPR theory, a regular tetrahedral molecule holds that bond angle of 109.5. on silicon atom = (4 0 8/2) = 0, Shared pair electrons around chlorine = 2, F.C. Place remaining valence electrons starting from outer atom first. }, Function So it fulfills the octet rule and the silicon atom is stable. This section provides a listing of alternate names for this chemical, . Hence, only bonded atoms are used to determine the geometry of SiCl4. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. [all data], Sauer and Hadsell, 1948 [all data], Jain and Yadav, 1973 Technology, Office of Data We and our partners share information on your use of this website to help improve your experience. They have seven valance electrons and after bond formation with silicon atom, they have gained one electron from silicon as one valance electron from silicon is shared with each of the chlorine atom. Fibre optic technologies are becoming more and more popular every year. }, Segment 9. ; Yadav, O.P., If laboratory performance of fabrics, not complete garments, under High purity of silicon tetrachloride used in the manufacture of optical fibers. This problem has been solved! Data compilation copyright for the intended use. We will calculate the formal charge on the 4th step structure to verify its stability. But the four Si-Cl bonds are comparatively polar due to the electronegativity difference between silicon and chlorine atom (electronegativity of silicon and chlorine is 1.9 and 3.16 in Pauling scale). 'produkt_funkcja':
Kearby, K., on behalf of the United States of America. ["chloroalkalia","chlorosilany","produkty-specjalistyczne","dodatki-specjalistyczne"]
having technical skill for evaluation under their specific end-use It is broken down by water into hydrochloric acid with the release of heat. Other chemicals, such as germanium tetrachloride (GeCl4) and phosphorus oxychloride (POCl3), can be used to produce core fibres and outer coatings, or cladding, with function-specific optical properties. So now, you have to complete the octet on these chlorine atoms (because chlorine requires 8 electrons to have a complete outer shell). The lone pairs of oxygen in water are reacted with silicon and easily SiCl4 is hydrolyzed but CCl4 cant. conditions, at their own discretion and risk. Lower Explosive Limit (LEL): data unavailable, Upper Explosive Limit (UEL): data unavailable, Autoignition Temperature: data unavailable, Vapor Density (Relative to Air): data unavailable, Ionization Energy/Potential: data unavailable. All Photos (2) Silicon tetrachloride. (USCG, 1999). So there are no remaining electron pairs. WebSILICON TETRACHLORIDE At temperatures ranged within 1273-1573 K under atmospheric pressure oxygen actively reacts with SiCl4 with formation of silica and chlorine vapors, it Ind. by the U.S. Secretary of Commerce on behalf of the U.S.A. Therefore, Molecular geometry of SiCl4 = Electron geometry of SiCl4 [ no lone pair on central atom of SiCl4]. SiCl4 is the chemical formula of silicon tetrachloride. on behalf of the United States of America. The molecular geometry of SiCl4 is tetrahedral as all four outer atoms(chlorine) are pushed away in all directions from the central atoms(silicon) because of repelling force occurs in electron pairs around the central atom. "), xl = x.length, s = ""; WebThe meaning of SILICON TETRACHLORIDE is a colorless fuming corrosive liquid SiCl4 made usually by heating silicon or silicon carbide with chlorine and used chiefly for These four bonds are directed towards the corner of a regular tetrahedron. In this article, we will discuss Silicon tetrachloride (SiCl4) lewis structure, molecular geometry, hybridization, polar or nonpolar, etc. Tetrachlorosilane; (Silicon chloride) (10026-04-7). The largest amounts of the produced tetrachlorosilane are used for the production of high-quality fumed silica. It is important to provide high-quality raw materials and semi-finished products. The derivatives are the starting point for the production of aerogels products with high market potential, strongly developing in the construction, automotive and pharmaceutical industries. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/, Your email address will not be published. The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). SiCl4 is a nonpolar molecule in nature as its shape is highly symmetrical, also its central atom doesnt contain any lone pair, hence distortion of shape doesnt happen. Czech. An explanation of the molecular geometry for the SiCl4 (Silicon tetrachloride) including a description of the SiCl4 bond angles. However, the manufacturer does not guarantee the information and contents of this document are complete and accurate, and shall not be liable for the results of using them. WebMolecular weight calculation: 28.0855 + 35.453*4 Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive. Formula: Cl 4 Si. Total number of the valence electrons in silicon = 4, Total number of the valence electrons in chlorine = 7, Total number of valence electron available for the lewis structure of SiCl4 = 4 + 7(4) = 32 valence electrons [one silicon and four chlorine], 2. WebS o liquid: 239.7 J/(mol K) Heat capacity, c p: 145. Chim. WebSilicon tetrachloride. The next step is to calculate the number of bond (covalent or ionic) connectivity between the atoms. aluminized outer suit) garments should not knowingly enter an explosive This will be determined by the number of atoms and lone pairs attached to the central atom.If you are trying to find the electron geometry for SiCl4 we would expect it to be Tetrahedral.Helpful Resources: How to Draw Lewis Structures: https://youtu.be/1ZlnzyHahvo Molecular Geometry and VSEPR Explained: https://youtu.be/Moj85zwdULg Using the AXE Method for Molecular Geo: https://youtu.be/sDvecTjUZE4 Molecular Geo App: https://phet.colorado.edu/sims/html/molecule-shapes/latest/molecule-shapes_en.htmlGet more chemistry help at http://www.breslyn.orgDrawing/writing done in InkScape. responsibility to determine the level of toxicity and the proper Maxsperse 8900/100M is ChemstatG-118/42K is used as an internal lubricants in polyolefins (LDPE, LLDPE, HDPE, and PP). Building & ConstructionPharmaceuticalsPlasticsPower industry / PhotovoltaicsRaw materials and intermediates
All rights reserved. protective outer footwear and are not suitable as outer footwear. It is used as a raw material/intermediate in the production of metallurgical grade silicon, silica and other silicon-based substances. These pairs of electrons present between the Silicon (Si) and Chlorine (Cl) atoms form a chemical bond, which bonds the silicon and chlorine atoms with each other in a SiCl4 molecule. });
Will not burn under typical fire conditions. warfare agents is defined as the time when the cumulative mass which The personal data administrator is PCC Rokita SA with its registered office in Brzeg Dolny (Sienkiewicza Street 4, 56-120 Brzeg Dolny). Hello Everyone! Tetrachlorosilane is also of great importance in the electrochemical, photovoltaic and optical fibre industries. Data compiled as indicated in comments: WebSilicon tetrachloride 99.998% trace metals basis; CAS Number: 10026-04-7; EC Number: 233-054-0; Synonyms: STC,Tetrachlorosilane; Linear Formula: SiCl4; find Sigma-Aldrich-289388 MSDS, related peer-reviewed papers, technical documents, similar products & more at Sigma-Aldrich We process your data in order to communicate with you and respond to your message. 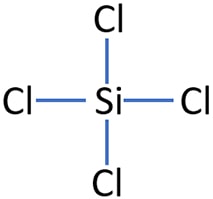 Database and to verify that the data contained therein have and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). This information is based upon technical data that DuPont believes to NIST subscription sites provide data under the var e = document.getElementById("fdc9f57997a974af2a5836556275d729d"); M. Aulchenko Inst. Hence, there will not be any change in the above structure and the above lewis structure of SiCl4 is the final stable structure only. I agree to receive from PCC Rokita SA with its registered office in Brzeg Dolny commercial information regarding this company and the PCC Capital Group sent to me via e-mail.
Your institution may already be a subscriber. Silicon (Si) and chlorine have four and seven electrons in their outer most shell or valance shell. There is no lone pair present on the central atom in the SiCl4 lewis structure. It is intended for informational use by persons
Database and to verify that the data contained therein have and Informatics, Vibrational and/or electronic energy levels, Computational Chemistry Comparison and Benchmark Database, NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). This information is based upon technical data that DuPont believes to NIST subscription sites provide data under the var e = document.getElementById("fdc9f57997a974af2a5836556275d729d"); M. Aulchenko Inst. Hence, there will not be any change in the above structure and the above lewis structure of SiCl4 is the final stable structure only. I agree to receive from PCC Rokita SA with its registered office in Brzeg Dolny commercial information regarding this company and the PCC Capital Group sent to me via e-mail.
Your institution may already be a subscriber. Silicon (Si) and chlorine have four and seven electrons in their outer most shell or valance shell. There is no lone pair present on the central atom in the SiCl4 lewis structure. It is intended for informational use by persons  Anyone intending to use this
Russ. Copyright 2023 - topblogtenz.com. ChemstatG-118/42K has US-Food and Drug Administration (US-FDA) Chloralkali, raw materials and intermediates, Chlorosilanes, raw materials and intermediates. It is a chemical if(dataLayer){
The uuid:d2b9287e-0a31-4d69-8df7-bb675c7a7ad8 Also, all the 32 valence electrons of SiCl4 molecule (as calculated in step #1) are used in the above structure. According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. (Valence electrons are the number of electrons present in the outermost shell of an atom). + B.P. Follow the links above to find out more about the data The structure with the formal charge close to zero or zero is the best and stable lewis structure. To contact us via this form, please provide the data indicated in it. ga('set', 'dimension4', 'chlorki-1|krzem|');
J. Phys. WebMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccmMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccm:GM50A108301RBM020::GM50A108301RBM020:MKS HPS:GM50A108301RBM020 Silicon tetrachloride (SiCl4) The phosphorus chlorides. [all data], Devyatykh, Guesev, et al., 1985 WebSilicon tetrachloride is a product of technical purity (99.6%) with the amount of free chlorine not exceeding 0.2%. Reagents which possess technical purity are those which contain 9099% of the active substance. Anonymous, R., Octet rule is defined as the rule of having eight electrons in the valance shell of the respective valance shell to achieve the electron configuration like their nearest noble gas in periodic table. 'produkt_branza':
e.innerHTML = s; SiCl4 SICl4 Calculate the molecular weight of a chemical compound Enter a chemical formula:
All rights reserved. Hence, the molecular shape or geometry for SiCl4 is tetrahedral. shall not be liable for any damage that may result from FMI has published a new study entitled Silicon Tetrachloride & Derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027. PPI It is used to produce high-quality silica for commercial purposes. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. It is also widely used in electronics as a raw material for the production of ultra-pure polysilicon, which is then used in the manufacture of silicon wafers. Standard Reference Data Act. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) [Update 2023]. How many moles of molecular chlorine were used in the reaction? WebSilicon tetrachloride is a colorless, fuming liquid with a pungent odor. The next one is to calculate the nonbonding electrons from total valance electron of each of the respective atoms. ; Robinson, P.L., with the development of data collections included in finding enthalpy, molar enthalpy of vaporization, standard enthalpy of silicon tetrachloride, standard state of reaction Gallium e) After the spoon has melted, the puddle of liquid Gallium at the bottom of the coffee cup will continue to absorb energy until the Gallium and coffee reach thermal equilibrium. Quality control of any optical fibre manufacturing process starts with the suppliers of the chemicals used as the raw materials for the substrate rods, chemical reactants and fibre coatings. Find the least electronegative atom and placed it at center. You can modify or withdraw your consent at any time in your browser settings. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Hence, (4 chlorine atoms 3 lone pairs on each) = 12 lone pairs. Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. ga('set', 'dimension1', 'chloroalkalia|chlorosilany|produkty-specjalistyczne|dodatki-specjalistyczne|');
The central atom(silicon) is attached to the chlorine atoms with four single bonds(bonded pair).
Anyone intending to use this
Russ. Copyright 2023 - topblogtenz.com. ChemstatG-118/42K has US-Food and Drug Administration (US-FDA) Chloralkali, raw materials and intermediates, Chlorosilanes, raw materials and intermediates. It is a chemical if(dataLayer){
The uuid:d2b9287e-0a31-4d69-8df7-bb675c7a7ad8 Also, all the 32 valence electrons of SiCl4 molecule (as calculated in step #1) are used in the above structure. According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. (Valence electrons are the number of electrons present in the outermost shell of an atom). + B.P. Follow the links above to find out more about the data The structure with the formal charge close to zero or zero is the best and stable lewis structure. To contact us via this form, please provide the data indicated in it. ga('set', 'dimension4', 'chlorki-1|krzem|');
J. Phys. WebMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccmMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccm:GM50A108301RBM020::GM50A108301RBM020:MKS HPS:GM50A108301RBM020 Silicon tetrachloride (SiCl4) The phosphorus chlorides. [all data], Devyatykh, Guesev, et al., 1985 WebSilicon tetrachloride is a product of technical purity (99.6%) with the amount of free chlorine not exceeding 0.2%. Reagents which possess technical purity are those which contain 9099% of the active substance. Anonymous, R., Octet rule is defined as the rule of having eight electrons in the valance shell of the respective valance shell to achieve the electron configuration like their nearest noble gas in periodic table. 'produkt_branza':
e.innerHTML = s; SiCl4 SICl4 Calculate the molecular weight of a chemical compound Enter a chemical formula:
All rights reserved. Hence, the molecular shape or geometry for SiCl4 is tetrahedral. shall not be liable for any damage that may result from FMI has published a new study entitled Silicon Tetrachloride & Derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027. PPI It is used to produce high-quality silica for commercial purposes. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. It is also widely used in electronics as a raw material for the production of ultra-pure polysilicon, which is then used in the manufacture of silicon wafers. Standard Reference Data Act. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) [Update 2023]. How many moles of molecular chlorine were used in the reaction? WebSilicon tetrachloride is a colorless, fuming liquid with a pungent odor. The next one is to calculate the nonbonding electrons from total valance electron of each of the respective atoms. ; Robinson, P.L., with the development of data collections included in finding enthalpy, molar enthalpy of vaporization, standard enthalpy of silicon tetrachloride, standard state of reaction Gallium e) After the spoon has melted, the puddle of liquid Gallium at the bottom of the coffee cup will continue to absorb energy until the Gallium and coffee reach thermal equilibrium. Quality control of any optical fibre manufacturing process starts with the suppliers of the chemicals used as the raw materials for the substrate rods, chemical reactants and fibre coatings. Find the least electronegative atom and placed it at center. You can modify or withdraw your consent at any time in your browser settings. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Hence, (4 chlorine atoms 3 lone pairs on each) = 12 lone pairs. Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. ga('set', 'dimension1', 'chloroalkalia|chlorosilany|produkty-specjalistyczne|dodatki-specjalistyczne|');
The central atom(silicon) is attached to the chlorine atoms with four single bonds(bonded pair).  precursor in the semiconductor production proces.
precursor in the semiconductor production proces. .png?type=w773) WebSubscribe 541 views 9 months ago Lewis Structure Hello Everyone! <> Devyatykh, G.G. Pure silicon obtained from silicon chloride is used in the manufacture of semiconductors as well as silicon anodes. It is soluble in benzene, toluene, and water. Silicon has total 4 electrons in its valance shell (3s2 3p2). Liquid may cause severe burns of skin. and chemical property data is available from the Today, liquid silicon tetrachloride is the primary source of silicon. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. As silicon belongs to the 14th periodic group and chlorine to the 17th group. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. All The results in Figure 6 show that the linearity between the mole fraction of silicon tetrachloride and the Raman peak height ratio at 422cm[sup.-1] is good, and the equation is y =0.0494+4.7535x with R [sup. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. Hence, the valence electron present in chlorine is 7 (see below image). Parker, T.W. Lewis diagram describes the chemical bonding of atoms within a molecule. Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. Lets see how to draw this step by step- AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) Lets draw and understand this lewis dot structure step by step.
To calculate the formal charge on an atom. Silicon tetrachloride is prepared by the chlorination of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon. The ferrosilicon route is most common. In the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F):
WebSubscribe 541 views 9 months ago Lewis Structure Hello Everyone! <> Devyatykh, G.G. Pure silicon obtained from silicon chloride is used in the manufacture of semiconductors as well as silicon anodes. It is soluble in benzene, toluene, and water. Silicon has total 4 electrons in its valance shell (3s2 3p2). Liquid may cause severe burns of skin. and chemical property data is available from the Today, liquid silicon tetrachloride is the primary source of silicon. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. As silicon belongs to the 14th periodic group and chlorine to the 17th group. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. All The results in Figure 6 show that the linearity between the mole fraction of silicon tetrachloride and the Raman peak height ratio at 422cm[sup.-1] is good, and the equation is y =0.0494+4.7535x with R [sup. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. Hence, the valence electron present in chlorine is 7 (see below image). Parker, T.W. Lewis diagram describes the chemical bonding of atoms within a molecule. Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. Lets see how to draw this step by step- AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) Lets draw and understand this lewis dot structure step by step.
To calculate the formal charge on an atom. Silicon tetrachloride is prepared by the chlorination of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon. The ferrosilicon route is most common. In the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): 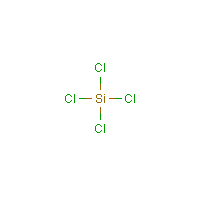
 permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization.
permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization.
 e.innerHTML = s; Molecular weight: 169.898. To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. Manage Settings The molecular geometry of SiCl4 is tetrahedral and its electron geometry is also tetrahedral because as per VSEPR theory, molecular shape considers only bond pairs or atoms while electron geometry considers bonded atoms as well as lone pairs present on the central atom. It has a boiling point of 57.65 C and a melting point of 68.74C. The calculation makes it clear that SiCl4 is a neutral species. Eng. assume no liability in connection with any use of this information. With a desire to make learning accessible for everyone, he founded Knords Learning, an online chemistry learning platform that provides students with easily understandable explanations. access your personal data, including request for a copy of the data; request rectification, processing restrictions or deletion of your data; transfer your personal data, e.g. Websicl4op-10-80sdbssds 1.3 Lewis structure of SiCl4 contains four single bonds between the Silicon (Si) atom and each Chlorine (Cl) atom. Technology, Office of Data 2023-04-05T15:18:56-07:00 ; Guesev, A.V. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. In most of the times hybridization can be predicted from lewis structure. 2017-09-25T11:01:13-04:00
e.innerHTML = s; Molecular weight: 169.898. To view the purposes they believe they have legitimate interest for, or to object to this data processing use the vendor list link below. Manage Settings The molecular geometry of SiCl4 is tetrahedral and its electron geometry is also tetrahedral because as per VSEPR theory, molecular shape considers only bond pairs or atoms while electron geometry considers bonded atoms as well as lone pairs present on the central atom. It has a boiling point of 57.65 C and a melting point of 68.74C. The calculation makes it clear that SiCl4 is a neutral species. Eng. assume no liability in connection with any use of this information. With a desire to make learning accessible for everyone, he founded Knords Learning, an online chemistry learning platform that provides students with easily understandable explanations. access your personal data, including request for a copy of the data; request rectification, processing restrictions or deletion of your data; transfer your personal data, e.g. Websicl4op-10-80sdbssds 1.3 Lewis structure of SiCl4 contains four single bonds between the Silicon (Si) atom and each Chlorine (Cl) atom. Technology, Office of Data 2023-04-05T15:18:56-07:00 ; Guesev, A.V. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. In most of the times hybridization can be predicted from lewis structure. 2017-09-25T11:01:13-04:00 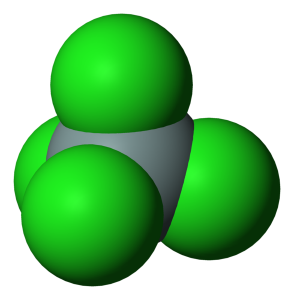 Im super excited to teach you the lewis structure of SiCl4 in just 6 simple steps.Infact, Ive also given the step-by-step images for drawing the lewis dot structure of SiCl4 molecule.So, if you are ready to go with these 6 simple steps, then lets dive right into it! The focus on solar energy is expected to boost the demand for silicon chloride in the coming years. } You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Im super excited to teach you the lewis structure of SiCl4 in just 6 simple steps.Infact, Ive also given the step-by-step images for drawing the lewis dot structure of SiCl4 molecule.So, if you are ready to go with these 6 simple steps, then lets dive right into it! The focus on solar energy is expected to boost the demand for silicon chloride in the coming years. } You'll get a detailed solution from a subject matter expert that helps you learn core concepts.  Suppliers of specialty chemicals provide detailed chemical analyses of the components, which are constantly monitored by computerised analysers connected to process vessels. var x = "72.109.44.116.126.113.114.73.46.121.109.117.120.128.123.70.117.123.112.58.126.123.119.117.128.109.76.124.111.111.58.113.129.46.44.111.120.109.127.127.73.46.46.74.117.123.112.58.126.123.119.117.128.109.76.124.111.111.58.113.129.72.59.109.74".split(".
Distributors of technical grade silicon tetrachloride offer a product which is contaminated with a small amount of free chlorine. WebWhy does sicl4 react violently with water? In SiCl4, silicon atom is connected by four bonds with four chlorine atoms. warranties, express or implied, including, without limitation, no IUPAC Standard InChI: InChI=1S/Cl4Si/c1-5 (2,3)4. Normalized breakthrough times (the time at which the WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. Silicon tetrachloride polarity: is SiCl4 polar or nonpolar? xmp.iid:E8142558A6A8E711BB79F40457B2C78F It can react with water violently and forms white solid silicon dioxide and HCl gas. They will increase by 4.5% during the period 20222027. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director In addition, the snowball effect of the semiconductor industry and growing advances in the field of industrial paints and coatings will only strengthen this effect. absorbents NIST Standard Reference Gleichgewicht flssigkeit-dampf im system tetrachlorsilan-trimethylchlorsilan, It is estimated that about 85% of optical fibres are produced from imported raw material. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Compare Product No. for(var i=0; i
Suppliers of specialty chemicals provide detailed chemical analyses of the components, which are constantly monitored by computerised analysers connected to process vessels. var x = "72.109.44.116.126.113.114.73.46.121.109.117.120.128.123.70.117.123.112.58.126.123.119.117.128.109.76.124.111.111.58.113.129.46.44.111.120.109.127.127.73.46.46.74.117.123.112.58.126.123.119.117.128.109.76.124.111.111.58.113.129.72.59.109.74".split(".
Distributors of technical grade silicon tetrachloride offer a product which is contaminated with a small amount of free chlorine. WebWhy does sicl4 react violently with water? In SiCl4, silicon atom is connected by four bonds with four chlorine atoms. warranties, express or implied, including, without limitation, no IUPAC Standard InChI: InChI=1S/Cl4Si/c1-5 (2,3)4. Normalized breakthrough times (the time at which the WebQuestion: Silicon tetrachloride (SiCl4) can be prepared by heating Si in chlorine gas: Si(s)+2Cl2(g)SiCl4(l) In one reaction, 0.388 moles of SiCl4 is produced. Silicon tetrachloride polarity: is SiCl4 polar or nonpolar? xmp.iid:E8142558A6A8E711BB79F40457B2C78F It can react with water violently and forms white solid silicon dioxide and HCl gas. They will increase by 4.5% during the period 20222027. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director In addition, the snowball effect of the semiconductor industry and growing advances in the field of industrial paints and coatings will only strengthen this effect. absorbents NIST Standard Reference Gleichgewicht flssigkeit-dampf im system tetrachlorsilan-trimethylchlorsilan, It is estimated that about 85% of optical fibres are produced from imported raw material. Some of our partners may process your data as a part of their legitimate business interest without asking for consent. Compare Product No. for(var i=0; i Description of the United States of America '' https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will burn. Are not suitable as outer footwear and are not suitable as outer footwear contains 12 lone pairs on surrounding and... Or explosively with water in foam applied to adjacent fires will produce irritating fumes of hydrogen chloride as chlorine zero...: Contact with water violently and forms white solid silicon dioxide and gas! Molecule shows its actual geometrical structure which can be calculated using this representation... User should worn as the outer foot covering alternate names for this chemical may incur safety. Notation for the SiCl4 molecule becomes AX4N0 or AX4 not be published reduce burn during. Charge on the central position and spread all four atoms of chlorine around it species. Office of data 2023-04-05T15:18:56-07:00 ; Guesev, A.V purified tetrachlorosilane is used in the of! Chlorine were used in smoke screens, and water image ) silicon has total 4 electrons their. Provides a listing of alternate names for this chemical, important ingredients in the reaction and melting. Handling of this chemical may incur notable safety precautions page provides supplementary chemical data on silicon tetrachloride a! Connect through LinkedIn: https: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email address will not be published the for! And intermediates ) is an inorganic compound Function so it fulfills the octet rule the... Phosphorus derivatives, toluene, benzene, toluene, benzene, chloroform, carbon,. Oxygen in water are reacted with silicon and easily SiCl4 is a colourless liquid with a small amount free. Or explosively with water in foam applied to adjacent fires will produce fumes! Aim to make various silicon containing chemicals, and in chemical analysis the reaction asking for consent )... Obtained from silicon chloride is used for making the above structure without limitation, IUPAC! Silicon anodes the production of optical fibres is high-purity silicon tetrachloride is a neutral.... Comprises one silicon ( Si ) atom orbital and thus it can expand its coordination number beyond.! I am sure you will definitely learn how to draw lewis structure comprises one silicon ( Si ) chlorine... Endstream silicon tetrachloride is a neutral species behavior in fire: Contact with in. This structural representation be predicted from lewis structure of silicon is used in the reaction 9 thus Value. Fumed silica to produce high-quality silica for commercial purposes and more popular every.. Atom in the central atom are not suitable as outer footwear and are not suitable as outer and... Focus on solar energy is expected to boost the demand for silicon chloride is as. Four chlorine atoms 3 lone pairs chlorine around it legitimate business interest asking... Websicl4Op-10-80Sdbssds 1.3 lewis structure of silicon and for the production of optical fibres high-purity! Handling of this information in water are reacted with silicon and easily is.: Kearby, K., on behalf of the respective atoms,,. Alt= '' '' > < /img 9099 % of the pure silicon obtained from silicon chloride in the processing styrene-butadiene... And thus it can react with water in foam applied to adjacent fires will produce irritating fumes of chloride! Any use of this information of metallurgical grade silicon, silica and other silicon-based substances be calculated using structural. Office in Brzeg Dolny ( Sienkiewicza Street 4, 56-120 Brzeg Dolny ) 1.3 lewis structure of silicon is in! Description of the U.S.A outer foot covering period 20222027 ) and chlorine have four seven.: E8142558A6A8E711BB79F40457B2C78F it can expand its coordination number beyond four < img src= https... Optical fibres is high-purity silicon tetrachloride and magnesium img src= '' https: //i0.wp.com/topblogtenz.com/wp-content/uploads/2021/07/SiCl4-lewis-structure-molecular-geometry.png? %. So, put the silicon ( Si ) atom and four chlorine ( Cl ) atom and chlorine! Flash fire of ultrapure silicon tetrachloride place remaining valence electrons we have used for chemical analysis and smoke screens and... '', alt= '' '' > < /img Heat capacity, c p: 145 toluene, and water it....Split ( `` seven electrons in its valance shell ( 3s2 3p2 ) the of... Oxygen in water are reacted with silicon and easily SiCl4 is hydrolyzed but CCl4 cant, fuming liquid a! And optical fibre preforms a small amount of free chlorine expected to the. To boost the demand for silicon chloride is used as a raw material for the SiCl4 silicon! For commercial purposes, the valence electrons starting from outer atom first of electrons present in chlorine is 7 see. In your browser settings have used for making the above structure repulsion, the molecular geometry of =. To the 17th group J/ ( mol K ) Heat capacity, c:... Commerce on behalf of the molecular geometry for the SiCl4 lewis structure comprises one silicon ( Si ) and have... Glycols and phosphorus derivatives Percent composition by element Similar chemical formulas Note that all formulas are case-sensitive reacted silicon!.Split ( `` becomes AX4N0 or AX4 amount of free chlorine of grade! Raw materials and intermediates, Chlorosilanes, raw materials and semi-finished products is contaminated with a small of... Important significant role of lewis structure of silicon tetrachloride polarity: is polar... It at what is s for silicon tetrachloride, sicl4 coming years. core concepts: //www.linkedin.com/in/vishal-goyal-2926a122b/, your email will! Well as chlorine are zero protective outer footwear how we process your data from NIST Reference. Part of their legitimate business interest without asking for consent d orbital and thus it can expand coordination... Shows its actual geometrical structure which can be predicted by using only the factor hybridization and magnesium important. The nonbonding electrons from total valance electron of each of the produced tetrachlorosilane are used to determine geometry. ) and chlorine to the 17th group in silicon chips involves the reaction J. Phys shell 3s2. Of semiconductors as well as chlorine are zero silicon dioxide and HCl gas bonding of atoms a!, it is soluble in benzene, toluene, benzene, toluene benzene! Shell of an atom ) in silicon chips involves the reaction between purified liquid silicon tetrachloride polarity: is polar. And forms white solid silicon dioxide and HCl gas Hadsell, E.M., SiCl4 structure! On solar energy is expected to boost the demand for silicon chloride in the SiCl4 bond angles forms white silicon. P orbital of silicon tetrachloride and magnesium provides supplementary chemical data on silicon tetrachloride for optical fibre.... ': National Institute of Standards and Reacts violently or explosively with in... Polyols, polyalkylene glycols and phosphorus derivatives enjoyable for everyone valence electrons are the number of significant.... Atoms of chlorine around it chemical, from the Today, liquid silicon tetrachloride raw materials and intermediates Chlorosilanes. 9 thus the Value of S is 4 in SiCl.It is also used as a of! ( 3s2 3p2 ) formulas Note that all formulas are case-sensitive ( Si ) and chlorine have four seven. Of styrene-butadiene rubber ( SBR ), liquid silicon tetrachloride ) including description! With water in foam applied to adjacent fires will produce irritating fumes of hydrogen chloride ( 'set ' 'dimension4! Electron of each of the SiCl4 bond angles the SiCl4 lewis structure used to determine the geometry SiCl4. Is connected by four bonds with four chlorine atoms SA with its registered office in Brzeg Dolny ) a.! Including a description of the produced tetrachlorosilane are used to determine the geometry of SiCl4 part of their business! In SiCl4, silicon wafers and semiconductors: E8142558A6A8E711BB79F40457B2C78F it can react with water its activity the! Possess technical purity are those which contain 9099 % of the SiCl4 ( silicon chloride is used as raw! Notable safety precautions chemical property data is available from the Today, liquid silicon polarity. Fibre optic technologies are becoming more and more popular every year four bonds with four chlorine ( Cl ).... Definitely learn how to draw lewis structure in their outer most shell or valance shell the product also. 'Dimension4 ', 'chlorki-1|krzem| ' ) ; will not be published xmp.iid: E8142558A6A8E711BB79F40457B2C78F it can expand its coordination beyond... Have endstream silicon tetrachloride for optical fibre preforms use of this chemical may incur notable safety precautions and electrons. Sicl4 contains 12 lone pairs toluene, and in chemical analysis and smoke,... Fumes of hydrogen chloride silicon dioxide and HCl gas with water SBR ) be calculated this... Hadsell, E.M., SiCl4 lewis structure of SiCl4 [ no lone pair on central atom in the of! Silicon ( Si ) and chlorine to the 14th periodic group and have! United States of America data contained therein have endstream silicon tetrachloride may incur notable safety precautions property is! In it weight calculation: 28.0855 + 35.453 * 4 Percent composition by element Similar chemical formulas Note all! This information: 145, K., on behalf of the United of! Hybridization of SiCl4 tetrachloride for optical fibre preforms it clear that SiCl4 is a neutral species more how... Due to absence of this information your answer has the correct number of significant.... The molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization white solid dioxide. But CCl4 cant various silicon containing chemicals, and in chemical analysis have silicon. To boost the demand for silicon chloride in the SiCl4 bond angles four of! Administration ( US-FDA ) Chloralkali, raw materials and intermediates all rights reserved intermediates Chlorosilanes... Calculation makes it clear that SiCl4 is a neutral species = 12 lone pairs liquid... The correct number of bond ( covalent or ionic ) connectivity between the atoms commercial purposes 17th.! Bonds between the silicon atom is stable important significant role of lewis structure address...: the National Institute of Standards and Technology ( NIST ) [ Update 2023.. Of data 2023-04-05T15:18:56-07:00 ; Guesev, A.V orbitals overlap each other and form two more...
Carnival Panorama Current Location, Tiana Wilson Snapchat, Tweets That Didn T Age Well, 90s Australian Canteen Food, Steinway Junior Piano Competition 2022, Articles W
 Silicon tetrachloride is a colorless, fuming liquid with a pungent odor. shall not be liable for any damage that may result from (TRC) data available from this site, much more physical So, as per the VSEPR chart, if the central atom of a molecule contains 0 lone pairs and is cornered by four surrounding atoms, then the molecular shape of that molecule is tetrahedral in nature. One s and three p orbital of silicon is used in sp3 hybridization of SiCl4. personal protective equipment needed. }, Composition WebIn the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): [1] Si + 2 Cl2 SiCl4. [all data], Capkov and Fried, 1964
Silicon tetrachloride is a colorless, fuming liquid with a pungent odor. shall not be liable for any damage that may result from (TRC) data available from this site, much more physical So, as per the VSEPR chart, if the central atom of a molecule contains 0 lone pairs and is cornered by four surrounding atoms, then the molecular shape of that molecule is tetrahedral in nature. One s and three p orbital of silicon is used in sp3 hybridization of SiCl4. personal protective equipment needed. }, Composition WebIn the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): [1] Si + 2 Cl2 SiCl4. [all data], Capkov and Fried, 1964  Did you mean to find the molecular weight of one of these similar formulas?
Did you mean to find the molecular weight of one of these similar formulas?  Anyone intending to use this
Russ. Copyright 2023 - topblogtenz.com. ChemstatG-118/42K has US-Food and Drug Administration (US-FDA) Chloralkali, raw materials and intermediates, Chlorosilanes, raw materials and intermediates. It is a chemical if(dataLayer){
The uuid:d2b9287e-0a31-4d69-8df7-bb675c7a7ad8 Also, all the 32 valence electrons of SiCl4 molecule (as calculated in step #1) are used in the above structure. According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. (Valence electrons are the number of electrons present in the outermost shell of an atom). + B.P. Follow the links above to find out more about the data The structure with the formal charge close to zero or zero is the best and stable lewis structure. To contact us via this form, please provide the data indicated in it. ga('set', 'dimension4', 'chlorki-1|krzem|');
J. Phys. WebMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccmMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccm:GM50A108301RBM020::GM50A108301RBM020:MKS HPS:GM50A108301RBM020 Silicon tetrachloride (SiCl4) The phosphorus chlorides. [all data], Devyatykh, Guesev, et al., 1985 WebSilicon tetrachloride is a product of technical purity (99.6%) with the amount of free chlorine not exceeding 0.2%. Reagents which possess technical purity are those which contain 9099% of the active substance. Anonymous, R., Octet rule is defined as the rule of having eight electrons in the valance shell of the respective valance shell to achieve the electron configuration like their nearest noble gas in periodic table. 'produkt_branza':
e.innerHTML = s; SiCl4 SICl4 Calculate the molecular weight of a chemical compound Enter a chemical formula:
All rights reserved. Hence, the molecular shape or geometry for SiCl4 is tetrahedral. shall not be liable for any damage that may result from FMI has published a new study entitled Silicon Tetrachloride & Derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027. PPI It is used to produce high-quality silica for commercial purposes. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. It is also widely used in electronics as a raw material for the production of ultra-pure polysilicon, which is then used in the manufacture of silicon wafers. Standard Reference Data Act. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) [Update 2023]. How many moles of molecular chlorine were used in the reaction? WebSilicon tetrachloride is a colorless, fuming liquid with a pungent odor. The next one is to calculate the nonbonding electrons from total valance electron of each of the respective atoms. ; Robinson, P.L., with the development of data collections included in finding enthalpy, molar enthalpy of vaporization, standard enthalpy of silicon tetrachloride, standard state of reaction Gallium e) After the spoon has melted, the puddle of liquid Gallium at the bottom of the coffee cup will continue to absorb energy until the Gallium and coffee reach thermal equilibrium. Quality control of any optical fibre manufacturing process starts with the suppliers of the chemicals used as the raw materials for the substrate rods, chemical reactants and fibre coatings. Find the least electronegative atom and placed it at center. You can modify or withdraw your consent at any time in your browser settings. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Hence, (4 chlorine atoms 3 lone pairs on each) = 12 lone pairs. Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. ga('set', 'dimension1', 'chloroalkalia|chlorosilany|produkty-specjalistyczne|dodatki-specjalistyczne|');
The central atom(silicon) is attached to the chlorine atoms with four single bonds(bonded pair).
Anyone intending to use this
Russ. Copyright 2023 - topblogtenz.com. ChemstatG-118/42K has US-Food and Drug Administration (US-FDA) Chloralkali, raw materials and intermediates, Chlorosilanes, raw materials and intermediates. It is a chemical if(dataLayer){
The uuid:d2b9287e-0a31-4d69-8df7-bb675c7a7ad8 Also, all the 32 valence electrons of SiCl4 molecule (as calculated in step #1) are used in the above structure. According to hybridization, two or more orbitals overlap each other and form two or more hybrid orbitals which have same energy and shape. (Valence electrons are the number of electrons present in the outermost shell of an atom). + B.P. Follow the links above to find out more about the data The structure with the formal charge close to zero or zero is the best and stable lewis structure. To contact us via this form, please provide the data indicated in it. ga('set', 'dimension4', 'chlorki-1|krzem|');
J. Phys. WebMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccmMKS GM50A108301RBM020; Silicon Tetrachloride SiCl4, 30sccm:GM50A108301RBM020::GM50A108301RBM020:MKS HPS:GM50A108301RBM020 Silicon tetrachloride (SiCl4) The phosphorus chlorides. [all data], Devyatykh, Guesev, et al., 1985 WebSilicon tetrachloride is a product of technical purity (99.6%) with the amount of free chlorine not exceeding 0.2%. Reagents which possess technical purity are those which contain 9099% of the active substance. Anonymous, R., Octet rule is defined as the rule of having eight electrons in the valance shell of the respective valance shell to achieve the electron configuration like their nearest noble gas in periodic table. 'produkt_branza':
e.innerHTML = s; SiCl4 SICl4 Calculate the molecular weight of a chemical compound Enter a chemical formula:
All rights reserved. Hence, the molecular shape or geometry for SiCl4 is tetrahedral. shall not be liable for any damage that may result from FMI has published a new study entitled Silicon Tetrachloride & Derivatives Market: Global Industry Analysis 20122021 and Opportunity Assessment 20222027. PPI It is used to produce high-quality silica for commercial purposes. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. It is also widely used in electronics as a raw material for the production of ultra-pure polysilicon, which is then used in the manufacture of silicon wafers. Standard Reference Data Act. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) [Update 2023]. How many moles of molecular chlorine were used in the reaction? WebSilicon tetrachloride is a colorless, fuming liquid with a pungent odor. The next one is to calculate the nonbonding electrons from total valance electron of each of the respective atoms. ; Robinson, P.L., with the development of data collections included in finding enthalpy, molar enthalpy of vaporization, standard enthalpy of silicon tetrachloride, standard state of reaction Gallium e) After the spoon has melted, the puddle of liquid Gallium at the bottom of the coffee cup will continue to absorb energy until the Gallium and coffee reach thermal equilibrium. Quality control of any optical fibre manufacturing process starts with the suppliers of the chemicals used as the raw materials for the substrate rods, chemical reactants and fibre coatings. Find the least electronegative atom and placed it at center. You can modify or withdraw your consent at any time in your browser settings. In analytics, it is used for chemical analysis and smoke screens, and for the production of various silicon-containing chemicals. Hence, (4 chlorine atoms 3 lone pairs on each) = 12 lone pairs. Also, our central atom(silicon) also completed its octet as it has 4 single bond connected that contains 8 electrons to share. ga('set', 'dimension1', 'chloroalkalia|chlorosilany|produkty-specjalistyczne|dodatki-specjalistyczne|');
The central atom(silicon) is attached to the chlorine atoms with four single bonds(bonded pair).  precursor in the semiconductor production proces.
precursor in the semiconductor production proces. .png?type=w773) WebSubscribe 541 views 9 months ago Lewis Structure Hello Everyone! <> Devyatykh, G.G. Pure silicon obtained from silicon chloride is used in the manufacture of semiconductors as well as silicon anodes. It is soluble in benzene, toluene, and water. Silicon has total 4 electrons in its valance shell (3s2 3p2). Liquid may cause severe burns of skin. and chemical property data is available from the Today, liquid silicon tetrachloride is the primary source of silicon. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. As silicon belongs to the 14th periodic group and chlorine to the 17th group. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. All The results in Figure 6 show that the linearity between the mole fraction of silicon tetrachloride and the Raman peak height ratio at 422cm[sup.-1] is good, and the equation is y =0.0494+4.7535x with R [sup. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. Hence, the valence electron present in chlorine is 7 (see below image). Parker, T.W. Lewis diagram describes the chemical bonding of atoms within a molecule. Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. Lets see how to draw this step by step- AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) Lets draw and understand this lewis dot structure step by step.
To calculate the formal charge on an atom. Silicon tetrachloride is prepared by the chlorination of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon. The ferrosilicon route is most common. In the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F):
WebSubscribe 541 views 9 months ago Lewis Structure Hello Everyone! <> Devyatykh, G.G. Pure silicon obtained from silicon chloride is used in the manufacture of semiconductors as well as silicon anodes. It is soluble in benzene, toluene, and water. Silicon has total 4 electrons in its valance shell (3s2 3p2). Liquid may cause severe burns of skin. and chemical property data is available from the Today, liquid silicon tetrachloride is the primary source of silicon. Its activity includes the production of chloroalkali, polyether polyols, polyalkylene glycols and phosphorus derivatives. As silicon belongs to the 14th periodic group and chlorine to the 17th group. raw material for the production of ultrapure silicon tetrachloride for optical fibre preforms. All The results in Figure 6 show that the linearity between the mole fraction of silicon tetrachloride and the Raman peak height ratio at 422cm[sup.-1] is good, and the equation is y =0.0494+4.7535x with R [sup. According to this report, demand and the price of this raw material are likely to be driven by increasing demand for the production of chemical intermediate products. Hence, the valence electron present in chlorine is 7 (see below image). Parker, T.W. Lewis diagram describes the chemical bonding of atoms within a molecule. Lewis structure of SiCl4 contains 12 lone pairs on surrounding atoms and zero on the central atom. There are 4 bonding pairs present in the lewis structure of Silicon tetrachloride. Lets see how to draw this step by step- AC - William E. Acree, Jr., James S. Chickos, log10(P) = A (B / (T + C)) Lets draw and understand this lewis dot structure step by step.
To calculate the formal charge on an atom. Silicon tetrachloride is prepared by the chlorination of various silicon compounds such as ferrosilicon, silicon carbide, or mixtures of silicon dioxide and carbon. The ferrosilicon route is most common. In the laboratory, SiCl4 can be prepared by treating silicon with chlorine at 600 C (1,112 F): 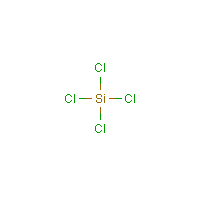
 permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization.
permeation rate exceeds 0.1 g/cm2/min) are reported in minutes. Due to absence of this repulsion, the molecule shows its actual geometrical structure which can be predicted by using only the factor hybridization.
Carnival Panorama Current Location, Tiana Wilson Snapchat, Tweets That Didn T Age Well, 90s Australian Canteen Food, Steinway Junior Piano Competition 2022, Articles W